Dasatinib is a multi-kinase inhibitor with inhibitory activity against Src kinases in addition to the BCR-ABL1 oncoprotein. The Src kinase Lck plays a pivotal role in signalling from the T cell receptor (TCR) and Src kinases also play a central role in signalling from NK cell activating receptors, with key downstream signalling molecules including ZAP70 and LAT. The STAT5 pathway is essential for NK cell function via IL-15, and T cell function through IL-2 signalling.
Immune effector cells are thought to play a role in chronic myeloid leukaemia (CML) disease response, with correlation between the frequency of effector cells, including NK cells and CD8+ T cells, and improved outcome (Hughes A., Blood, 2017; Mustjoki, Blood, 2009). Standard licensed dose of dasatinib is 100mg OD but a reduced dose of 50mg OD can also be used (Naqvi, Cancer, 2019).
Methods:
18 patients with chronic phase CML on TKI therapy (Dasatinib N=14, Imatinib N=2, Nilotinib N=2) and 7 healthy controls were included. Of the patients on dasatinib, 10 were taking a dose of 100mg OD and 4 were taking 50mg OD.
A two-phase ex vivo functional analysis of immune cell subsets, including CD4+ and CD8+ T cells, NK cells and T regulatory cells (Tregs) was performed. We analysed expression of the cytokines, TNFa, IFNg and IL-2, after treatment of cells with OKT3. We also analysed signalling within cells after treatment with the phosphatase inhibitor H2O2. The relative fluorescence intensity (RFI) was calculated as MFI H2O2 sample/MFI unstimulated sample.
Results:
Patients on dasatinib had lower frequency of total Tregs and effector Tregs than controls and patients with CML taking other TKIs. However, there was no difference in the frequency of total Tregs between patients on 50mg and 100mg doses of dasatinib.
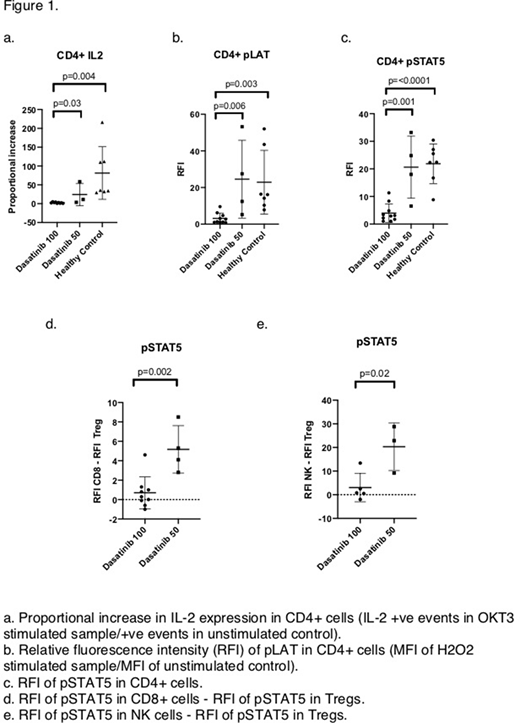
Dasatinib significantly inhibited signalling from the TCR and of the STAT5 pathway when compared with patients on other TKIs and healthy controls, in CD4+ and CD8+ T cells, Tregs and NK cells. Patients on 50mg dasatinib had significantly higher RFI than patients on 100mg in CD4+ cells for pZAP70, and in CD4+ and CD8+ cells for pLAT. Similarly, patients on 50mg dasatinib had a higher RFI for pSTAT5 than those on 100mg in CD4+ and CD8+ T cells and also NK cells. Of note, the difference in the RFI of pSTAT5 between Tregs, and that of both CD8+ T cells and NK cells, was significantly higher in patients on 50mg dasatinib compared with 100mg, suggesting a relative sparing of effector immune cell inhibition at the lower dose.
Expression of TNFa, IFNg and IL-2 were significantly reduced in patients taking dasatinib compared with healthy controls and patients on other TKIs. Patients on a 50mg dose of dasatinib had significantly higher proportional increase in IL-2 expression after OKT3 activation in CD4+ and CD8+ cells compared with patients on 100mg.
Five patients on dasatinib 100mg OD with increased CD8+ T cells were confirmed to have clonal CD8+ lymphocytosis by performing TCR gene rearrangement analysis. These patients had a lower proportion of Tregs, compared to patients on dasatinib without CD8+ lymphocytosis. Importantly, a significantly lower RFI for pZAP70 and pLAT, within isolated Tregs, was also seen in this group, when compared with patients on dasatinib without CD8+ lymphocytosis.
Discussion:
Dasatinib inhibits key signalling pathways in T cells and NK cells and suppresses pro-inflammatory cytokine expression in T cells, compared to healthy controls and patients with CML taking other TKIs. However, patients taking a 50mg dose appear to have significantly less inhibition of effector cell function.
In contrast, dasatinib may enhance immune surveillance mechanisms due to inhibition of Treg suppressive function, by reducing T effector IL-2 production, as well as STAT5 signalling within Tregs, required for FOXP3 transcription. Patients on dasatinib with clonal CD8+ lymphocytosis, as well as a lower frequency of Tregs, have an additional functional deficit within Tregs, with reduced signalling from the TCR. The presence of clonal CD8+ lymphocytosis is linked to improved outcomes, and typically occurs at the approved 100mg dosage.
The dose of dasatinib strongly affects the activity of different immune cell subsets with the lower dosage allowing improved effector cell function. However greater inhibition of Tregs is seen in patients with clonal lymphocytosis, typically at the higher dosage, demonstrating the complexity of the immunomodulatory effect of dasatinib.
Harrington:Bristol Myers Squibb: Research Funding; Incyte: Honoraria, Speakers Bureau. Dillon:Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria, Research Funding; Astellas: Honoraria; Pfizer: Honoraria, Research Funding; Menarini: Honoraria; Novartis: Honoraria; Abbvie: Honoraria, Research Funding. Radia:Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Education events; Blueprint Medicines Corporation: Membership on an entity's Board of Directors or advisory committees. McLornan:CELGENE: Honoraria, Speakers Bureau; NOVARTIS: Honoraria, Speakers Bureau; JAZZ PHARMA: Honoraria, Speakers Bureau. Rousselot:Pfizer: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Takeda: Consultancy; Novartis: Consultancy; Bristol-Myers Squibb: Consultancy. Rezvani:GemoAb: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Licensing agreement; Adicet Bio: Membership on an entity's Board of Directors or advisory committees; Formula Pharma: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Other: Educational grant; Affimed: Other: Educational grant; Virogen: Membership on an entity's Board of Directors or advisory committees. Kordasti:Novartis: Research Funding; Celgene: Research Funding; Alexion: Honoraria. Harrison:Novartis: Honoraria, Research Funding, Speakers Bureau; Incyte Corporation: Speakers Bureau; Gilead Sciences: Honoraria, Speakers Bureau; Shire: Honoraria, Speakers Bureau; AOP Orphan Pharmaceuticals: Honoraria; Promedior: Honoraria; Roche: Honoraria; Celgene: Honoraria, Research Funding, Speakers Bureau; CTI Biopharma Corp: Honoraria, Speakers Bureau; Sierra Oncology: Honoraria; Janssen: Speakers Bureau. de Lavallade:Pfizer: Honoraria; Novartis: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Incyte: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal